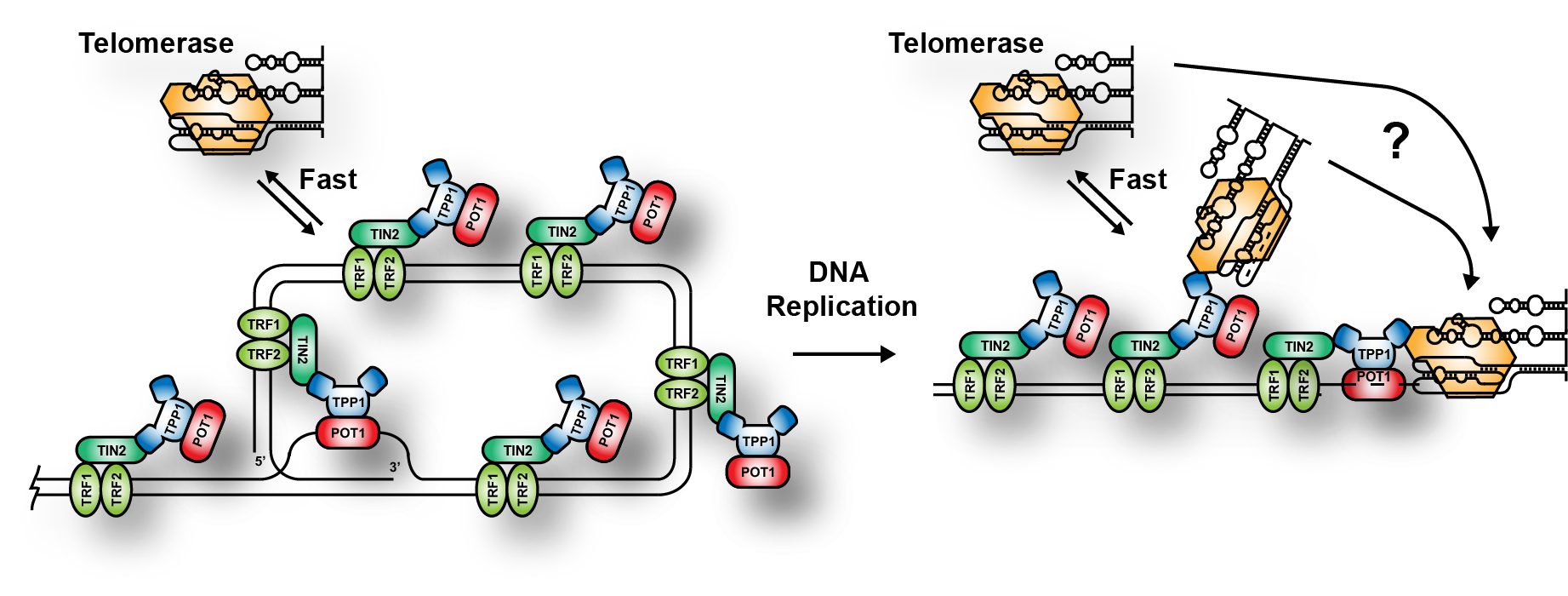
Telomerase recruitment to telomeres
Telomerase is recruited to telomeres via a direct interaction of the telomerase reverse transcriptase protein TERT and the shelterin complex component TPP1. We have demonstrated that telomerase forms two types of interactions with telomeres: Fast probing interactions, and long-static interactions, which likely represent telomere elongation events. To understand telomere length homeostasis we have two address two critical questions. How is the formation of long-static interactions regulated by individual telomeres, and how many nucleotides does telomerase add to the telomere during a single elongation event? Both of these question along with many others are the subject of ongoing research projects in the lab.
Telomerase Catalysis
Telomerase is a unique RNA containing reverse transcriptase. It adds telomeric repeats to the chromosome end by copying the template sequence contained in its RNA subunit. A unique aspect of telomerase is that it can translocate the substrate relative to the template sequence to processively synthesize multiple telomeric repeats in a single association event. Understanding the biochemical properties of telomerase is critical to building a quantitative model of telomere length homeostasis. We use a combination of biochemical and single-molecule approaches to analyze all aspects of the telomerase catalytic cycle. Most recently we have started using high-resolution optical tweezers in collaboration with Matt Comstock at MSU to define the molecular mechanism of processive telomerase catalysis.